Abstract
Relapsed and refractory (R/R) multiple myeloma patients have very poor prognosis, especially when they present extramedullary lesions. Currently, chimeric antigen receptor-modified T cells (CAR-T) for lymphoproliferative diseases such as B-precursor acute lymphoblastic leukemia and B-cell lymphoma have yielded encouraging therapeutic effects. However, clinical study on the efficacy of CAR-T in multiple myeloma is just at beginning. B cell maturation antigen (BCMA) displays restricted expression on plasma cells and is regarded as a potential therapeutic target in myeloma patients. In this one arm trial approved by the IRBs of the participating hospitals, bi-epitope targeting CAR-T with specificity against BCMA (LCAR-B38M) was used to treat R/R multiple myeloma. As of 2017-7-31, 5 patients (RJ01-03, CZ01-02, including 2 females and 3 males; average age: 51 years) were enrolled in the study. Their Ig subtypes were: IgG lamda in 2 cases, IgG kappa in 2 cases and IgD lamda in one case. Relapse occurred in all patients after treatment with classical chemotherapy, immunomodulatory drugs and proteosome inhibitors, 3 of them had also received autologous hematopoietic stem cell transplantation. Plasma cells accounted for 3% in one case (RJ03) and 22 to 69% in 4 cases among BM cells. BCMA was positive on plasma cells in all cases. Plasma M-proteins were at abnormally high levels (11.52-64.53g/L) in all these cases, and free light chains were positive in 4 cases but negative in the case RJ03. Of note, two patients exhibited extramedullary lesions. One bore plasmacytoma on the forehead and the other presented extramedullary involvement in the lower jaw, chest skin and liver. Interphase fluorescence in situ hybridization (FISH) detected high risk chromosome abnormalities in 3 cases, including one with 1q21 amplification, one with TP53 deletion, and one carried both of these two abnormalities. Split FISH signal of IGH was detected in 2 cases, though not juxtaposing to the common known high risk partner genes.
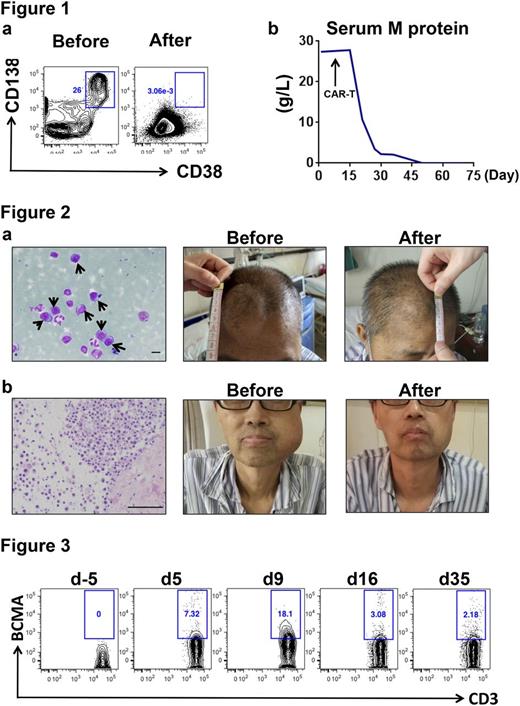
Patients underwent leukopheresis to collect T-cells for the preparation of BCMA-specific CAR-T. Pre-CAR-T treatment was performed with fludarabine (25mg/m2) and cyclophosphamide (250mg/m2) daily for 3 days (d-5~d-3). A dose of 0.17 or 1.05×106 (median=0.62) CAR-T cells per kilogram of body weight was intravenously infused on three days (d0, d2, and d6). At one month after CAR-T treatment, all 5 patients achieved an objective response, 1 achieving immunophenotypic complete remission, 1 reaching very good partial remission and 3 obtaining partial remission. Figure 1 shows the return to negativity of BM minimal residual disease and serum M protein in the case RJ01 at two months post CAR-T. Notably, large subcutaneous masses seen in two patients (RJ02, RJ03) were significantly reduced after treatment (Figure 2a and 2b). Peripheral blood CAR-Ts were consistently detected after infusion (Figure 3). The most common adverse effects were the high fever and hypotension attributable to cytokine release syndrome (CRS) and were manageable with nonsteroidal anti-inflammatory drugs (NSAIDs) or tocilizumab. The fluid was supplemented to maintain the blood pressure in case of need. No dose-limiting toxicities or treatment-related deaths were observed. Therefore, BCMA-specific CAR-T represents a promising safe therapeutic approach for R/R multiple myeloma, including cases with severe extramedullary invasions.
Figure 1. Minimal residual disease (MRD) and serum M protein in patient RJ01. (a) Flow cytometry detection of bone marrow MRD (CD138+CD38+CD56+CD19-) before and two months after CAR-T therapy. (b) The change of plasma M protein concentration after CAR-T infusion. The final assessment was on day 65 post CAR-T.
Figure 2. Subcutaneous masses reduction in patients with extramedullary plasmacytoma. (a) A large number of immature plasma cells are seen on the smears obtained from the mass on the forehead of the case RJ02 (black arrows). On day 20 post CAR-T infusion, the plasmacytoma on the forehead was remarkably reduced. Scale bar=10µm. (b) The histopathology reveals that the mass of the lower jaw in the case RJ03 was a plasmacytoma, which was largely reduced on day 23 post CAR-T. Scale bar=100µm.
Figure 3. CAR-T cells in peripheral blood of the patient RJ02. Flow cytometry shows the percentage of BCMA specific CAR-T cells in CD3 positive peripheral T cells. The time course is indicated as days (d) after CAR-T infusion.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal